Bioavailability Enhancement for Oral Solid Dosage Forms
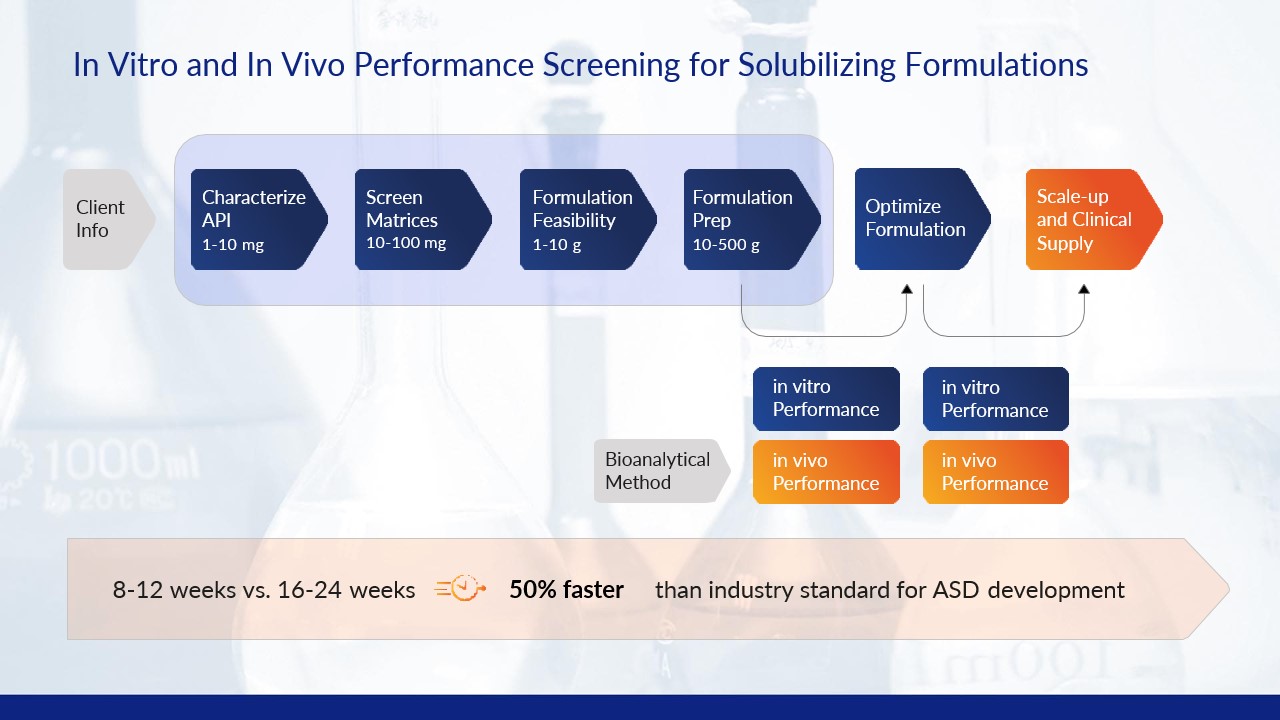
Our systematic approach screens formulations with amorphous solid dispersion (ASD) technologies to ensure the drug candidate with the highest oral bioavailability is selected for further development and/or manufacturing.
In silico computer modelling
In vitro discriminant analysis
In vivo ADME and PK studies
Stability studies
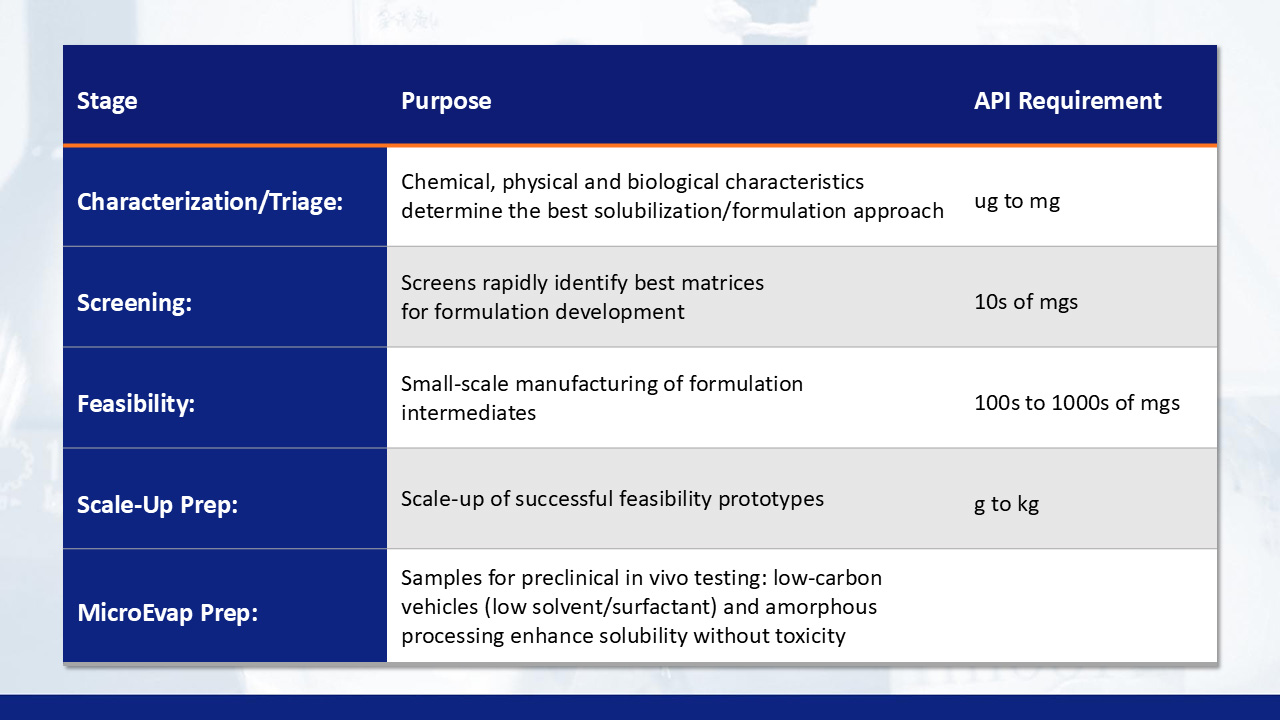
Formulation screening
Process scale-up
cGMP manufacturing
CTM supply and clinical manufacturing
Specializations include lipid-based solution and suspension, self-microemulsifying drug delivery system (SMEDDS), nanosuspension and micronization
ASD Screening Studies: in silico and in vivo
BioDuro-Sundia ASD screening studies leverage in silico methods based on the Flory-Huggins solution theory to select drug-polymer combinations with the highest chance of miscibility based on estimated solubility parameters and potential for polar, dipole and ionic interactions. Subsequent in vivo PK studies determine drug-polymer combinations with the highest oral bioavailability.
ASD Stability Studies: SDD and HME
Next, ASD stability studies utilize small-scale spray dried dispersion (SDD) and/or hot melt extrusion (HME) to characterize drug-polymer combinations for amorphous content determination (XRPD), thermal properties (mDSC and TGA), assay/related substance and in-vitro non-sink dissolution before being staged in ICH accelerated stability chambers. The ASDs with the highest stability proceed to further in vivo PK studies and selected for process scale-up and manufacturing.
ASD Process Scale-Up and Manufacturing
BioDuro-Sundia ASD process development and cGMP manufacturing capabilities enable downstream process and manufacturing of finished oral solid dosage forms (tablets and capsules). The process of SDD and/or HME is scaled-up for engineering process development. Finally, cGMP manufacturing, packaging, labeling (open or blinded) and clinical site distribution completes full-service supply of clinical trial material (CTM).
Contact Us
Click here or email hello@bioduro-sundia.com to get in contact with a BioDuro-Sundia representative. By combining in silico computer modeling, in vitro screening and in vivo PK with cGMP manufacturing, our unique approach optimizes ASDs for high oral bioavailability.