


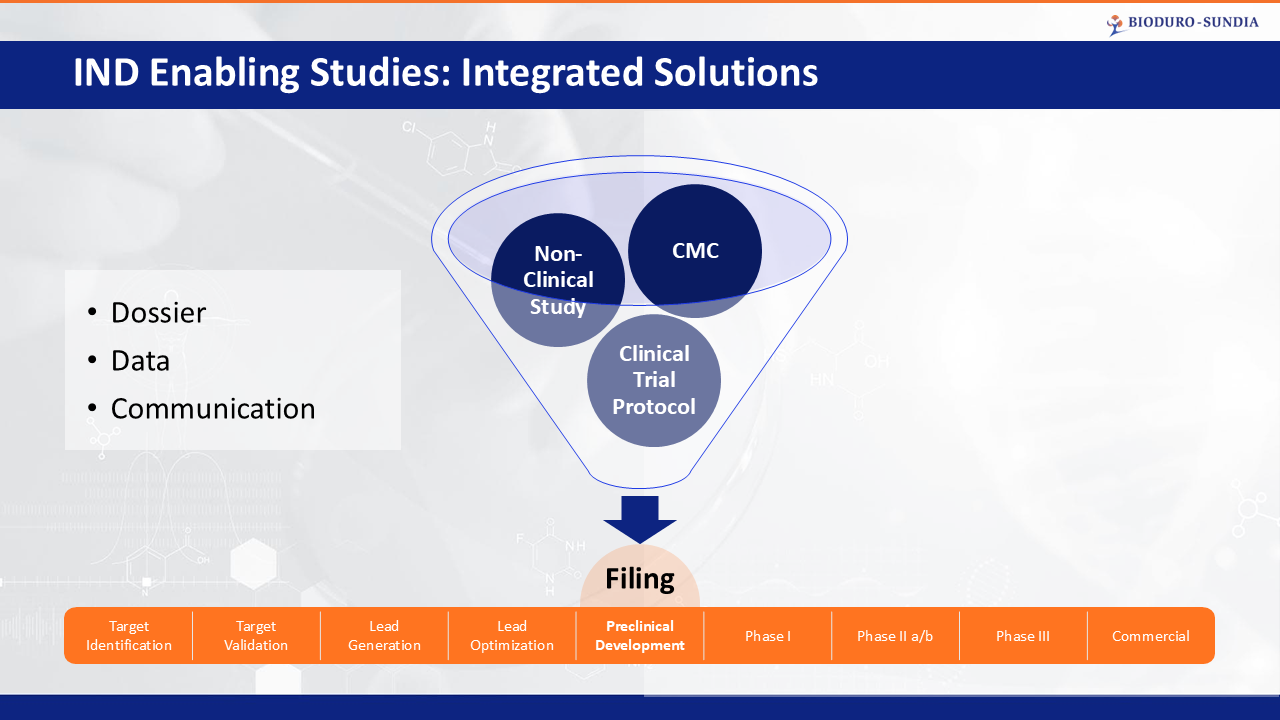
Provide a “one stop shop” service for IND filing
Provide clients with a comprehensive strategy, fulfilling the registration requirements, that lead the project to success
Extensive project management and IND submission experience develops vision and strategy to meet all your project's milestones
Dedicated preclinical project teams to help you succeed
Industry-leading integrated preclinical development expertise
Strict IP management and mature development procedure
Solution design: Regulatory consultation, project evaluation, strategy design
Research Service: Pharmacology (API, Formulation), Pharmacodynamics, DMPK, Toxicology
Integrated Service: Budget evaluation, time planning, project management, and coordination of functional teams across various modules
Registration Application: Write and compile CTD documents, submit registration applications, and communicate with regulatory authorities
Provide drug regulation and drug registration consultation
Provide project evaluation and risk analysis
Design and provide registration application strategy
Develop overall project scope, estimate project cost, plan project timeline, and ensure regulatory compliance
Active Pharmaceutical Ingredient (API)
Formulation
Pharmacodynamics (DMPK)
Pharmacology
Toxicology (Out-sourced)
Supervise research proposals to ensure that it meets the requirements of the overall scope.
Direct project direction, progress, quality, and budget. Make quick adjustments to save time and cost. Coordinate functional teams for data and to ensure integrity and compliance
Organize phase audits, prepare reports, and assist clients in risk assessment reports
Manage samples and documents for regulatory compliance
Manage outsourcing cooperation
Ensure the research process and research results meet the requirement of the registration application
Research summary & CTD document writing
IND documents preparation and compiling
Communication with the regulatory authorities
IND submission
Site inspection preparation